Thiacloprid was banned in 2020 because of its toxicity to the unborn, and concerns about the contamination of groundwater with carcinogenic metabolites. Thiacloprid is also highly toxic to bees and other pollinators. While EU law foresees that citizens and the environment should not be exposed to substances that are classified as 'Toxic to reproduction category 1B', the Commission attempted to give derogations for imported food, raising up to 1000 fold the exposure of citizens. Food grown with this chemical would be imported, putting EU farmers in an unfair competition. With this move, the Commission would give its consent to continue the use of this dangerous chemical in third countries jeopardising the health of local communities and biodiversity.
Today, 386 Members of the European Parliament opposed the Commission proposal while 186 supported it. In its proposal, the Commission planned to lower the Maximum Residue Level of thiacloprid in certain foodstuffs, while giving exceptions to imported food.
Salomé Roynel, a policy officer at PAN Europe said: “It took the Commission many years to ban this dangerous pesticide. And now it tried to maintain incredibly high levels in food like tea or strawberries. The use of this health- and bee-harming pesticide would continue elsewhere, while the health of EU consumers, particularly pregnant women and babies, would also be put at risk. We applaud the Members of the European Parliament for stepping in to prevent this from happening”.
Thiacloprid was classified as 'Toxic to reproduction category 1B' in 2015 by the European Chemicals Agency. The pesticide legislation prohibits any contact of citizens with such pesticides[1] and no residues should be detected in food. It was banned only in 2020, and in Fall 2023, the Commission proposed to Member States a new regulation to delete the Maximum Residue Levels (MRLs) in food (to the default detection limit), except for a series of imported foodstuffs.
Angeliki Lyssimachou, Head of Science and Policy said: “The Commission is incredibly slow in protecting its citizens against pesticides. It should follow EU law and ensure that the approval of such substances is withdrawn immediately after their classification as reprotoxicant, in order to protect pregnant women, babies and children. It’s outrageous that they took more than 8 years to finally act.”
The Commission grounds these 'import tolerances' on the Maximum Residue Levels Regulation[2]. Import tolerances are meant to allow the import of foodstuff from third countries with residues of pesticides that are not authorised in the EU. But only when there is no public health concern. In the meantime, the Pesticide regulation foresees that the public should not be exposed to carcinogens, mutagens, reprotoxicants and endocrine disruptors.
Angeliki Lyssimachou concluded: “The Commission’s double standards practice is clearly immoral. On the one hand we ban this highly toxic pesticide in the EU to protect our population and ecosystems. On the other hand the Commission wants to continue importing food grown elsewhere with this very same toxic chemical, knowing it’s putting the health of local communities and environmental resources at great risk. Today, the Parliament's block is a clear message: End this double standard for all hazardous pesticides banned in the EU.”
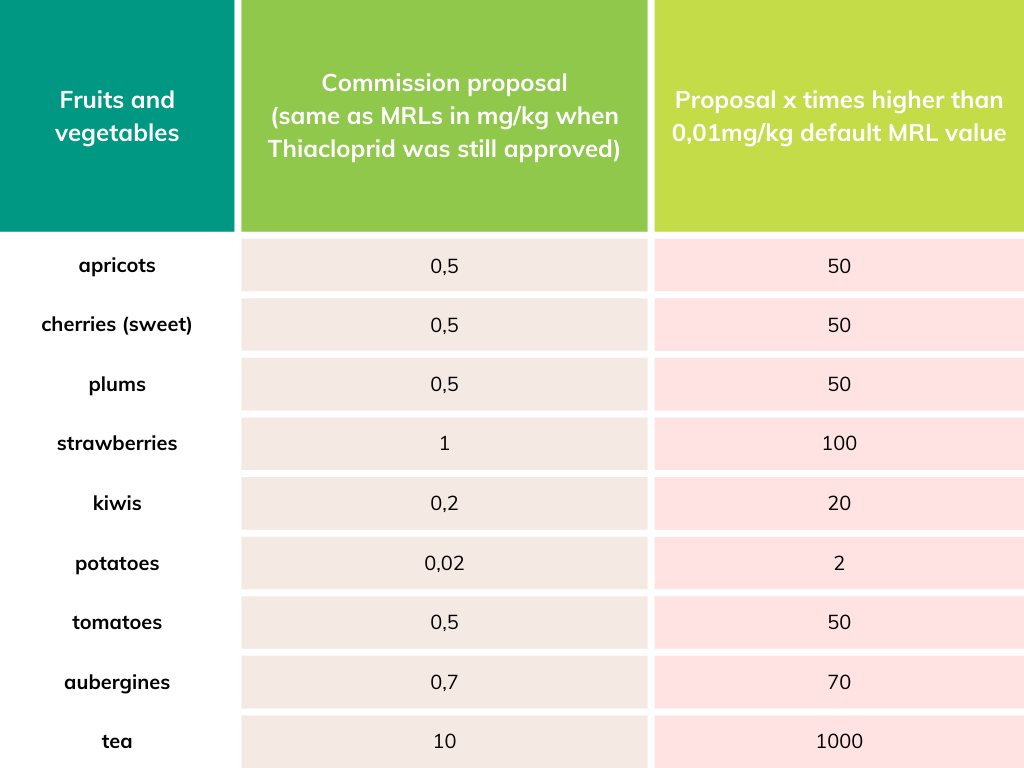
See for more background Should we allow residues of EU-banned Thiacloprid in imported food?
Contact: Salomé Roynel, Policy Officer on Pesticide Risk assessment: salome [at] pan-europe.info, +33 7 86 39 72 74
Notes:
[1] §3.6.4 from Annex II of regulation (EU) 1107/2009
[2] Regulation (EU) 396/2005
